Diving’s favourite Physicist Friends
By Gemma Smith
For many new divers, physics and the theory of gas laws may initially seem a little dry. It’s not as exciting as actually getting in the water, is it!? Actually, these various gas laws constitute one of the most interesting and relatable aspects of diving theory. Over the last centuries, several physicists have transformed our understanding of the behaviour of gases. This has allowed our diving sport to grow exponentially, and become infinitely safer. Many people initially balk at the thought of having to ‘go back to school’ to brush up on their diving physics. What you’ll soon discover though is that on a practical level it is much easier than you might think. Let us start by looking at some of our most inspiring and cutting-edge physicist friends over the years. Even more importantly, let us see how their discoveries affect our bodies and equipment on every dive we make.
Boyle’s Law
Named after Robert Boyle (1627-1691), this Law can also sometimes be referred to as the Boyle- Marriotte Law. This is in honour of the French physicist Edme Marriotte(1620-1684). Interestingly, he actually discovered this same gas law at around the time as Boyle did. In the diving world, it is more commonly known simply as ‘Boyle’s Law’ though.
At its most basic level, Boyle’s Law states:
At a constant temperature, the volume of a gas varies inversely with the pressure. The density of a gas varies directly with pressure.
Now that is all well and good, but what on earth does it actually mean? Basically, it is saying that when the surrounding pressure increases, the volume of the gas will decrease. The same amount of gas will take up less space. This is dependant on the temperature staying constant. On the flip side, as the surrounding pressure increases, the density of the gas will also increase. Once you get your head around these two aspects of Boyle’s Law you will see that it affects all divers, at all times, when underwater. For example:
- Why do you use more gas at depth than you do when diving shallow?
- Why do you need to equalise air spaces, such as ears, mask, and sinuses on descent?
- Why do you have to vent air from your BCD/wing on ascent, in order to maintain a slow and controlled speed?
- Why do you need to add more air to your drysuit or wing whenever you dive deeper, in order to maintain neutral buoyancy?
- Why is the most important rule in scuba diving is to never, ever hold your breath?
The answers to all of these examples are directly related to Boyle’s Law. He really is the granddaddy of diving physics, and someone we should remember every time we submerge.
Dalton’s Law
Now I have to admit I have a soft spot for John Dalton (1766-1844). When I first started out ‘technical’ diving, his formula was the backbone of my learning. Dalton’s Law is something I still make use of on every single dive I do, particularly in the planning stages. It says:
The total pressure exerted by a mixture of gasses is equal to the sum of the pressures that would be exerted by each of the gases if it alone were present and occupied by the total volume.
On first glance, this looks confusing, but it is actually very simple. All it is saying is that as pressure increases, the partial pressure of all the separate gases in a mix will also increase. So let’s look at this in more detail.
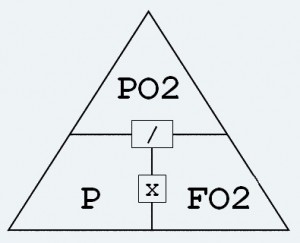
Air is made up of roughly 79% nitrogen and 21% oxygen. At the surface, at 1 bar of pressure, the partial pressure of nitrogen will equate to 0.79. The partial pressure of oxygen will be 0.21. Easy. So what happens when the pressure increases (such as when we go diving)? Imagine going down to 20m/66ft. The pressure increases one atmosphere of pressure absolute (ATA) for every 10m/ 33ft descended. That means at 20m/66ft the pressure will be 3 bar. As we have seen, as the pressure increases the partial pressure of the different components of a gas mixture will also increase. So if you are breathing air at 20m/66ft, the partial pressure of nitrogen would be 2.37 (0.79 multiplied by 3). The partial pressure of oxygen would be 0.63 (0.21 multiplied by 3). Simple! Dalton worked all this out and devised ‘Daltons Triangle’. This formula shows the relationship between partial pressure, the fraction of oxygen in a gas mix, and ambient pressure. This has been to the benefit of every diver in the world. It allows the calculation of the best oxygen content of a gas mix. It allows us to work out the maximum operating depth of certain gas mixes. The same law applies to blending trimix, heliox, and any other mixed gases you may use. It is a formula that you will probably make use of again and again for the rest of your diving journey.
Henry’s Law
- William Henry (1774-1836) was a physician from England. In the early 19th Century he formulated the concept of ‘Henry’s Law’. This law says:
The amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas. This is in equilibrium with that liquid. All this assumes that the temperature stays constant.
At its heart, this Law relates to two simple concepts. The idea that the greater the pressure you are under, the more gas that you will absorb into your tissues. This is alongside the finding that from an absorption aspect, temperature plays an important role. The warmer you are the less gas will remain in a soluble form in your body. The cooler you are, the more gas will remain in solution in your tissues.
So how does this relate to us as divers?
In simple terms, what this means is that when the ambient pressure around us increases due to depth, the partial pressure of oxygen and nitrogen in the body will also increase. There will be more molecules of each gas dissolved in your blood and tissues. This has a big impact on decompression issues for divers. All the gas we absorb during the dive then obviously needs off-gassing. It is vital we do this in a slow and controlled manner. This is why a steady and controlled ascent is so important. It is also why completing decompression stops on certain dive profiles is so critical.
Charles’ Law
Last, but definitely not least, we come to Charles. This Law has its name in honour of Jacques Alexandre Cesar Charles (1746-1823), who first made note of this theory in 1801. Charles’ Law says that:
At a constant volume, the pressure of a gas varies directly with absolute temperature
This Law is the most self-explanatory so far. Simply, when the volume of a gas fill stays constant the pressure will increase when warm, and decrease when cold. Charle’s Law explains why, if we get our cylinders filled on a hot day and then go dive in cold water, our starting pressure will drop. For example, it is 15 degrees Celsius air temperature outside, and you go to get your tanks filled. An 11-litre cylinder filled to 200 bar means you have 2200 litres of gas available. The water you are diving in is 8 degrees. When you check your starting pressure at the beginning of your dive you see you have less than expected! This is Charle’s Law in action. This is also why it is important not to leave a freshly filled tank in the trunk of a hot car. The pressure will only increase further, and possibly risk damage. This theory also explains why some gas filling stations at dive shops will place tanks in cold water prior to filling. This is to prevent your cylinders overheating.
Originally, none of these formulated concepts were considered for a diving audience. They were simply investigations into the nature and behaviour of gases. However, these various scientific pioneers have turned out to be some of scuba diving’s favourite physicist friends. It is through the knowledge and practical understanding of these various Laws of Physics that we as divers can be helped to stay safe, whatever the type of diving we may be undertaking.









 Natalie L. Gibb
Natalie L. Gibb
Leave a Reply
Want to join the discussion?Feel free to contribute!